WHAT IS A COVID-19 VACCINE?
The COVID-19 vaccine is a product that helps your body protect itself against the virus that causes COVID-19 infection. The vaccine “teaches” your body’s immune system how to make antibodies specifically against the virus that causes COVID-19. If you come into contact with the virus, these antibodies give you a better chance of fighting it off and not becoming infected. The vaccine is given to you as an injection.
Experts continue to study changes in both the virus and vaccines to find the best protection. Three vaccines are now available:
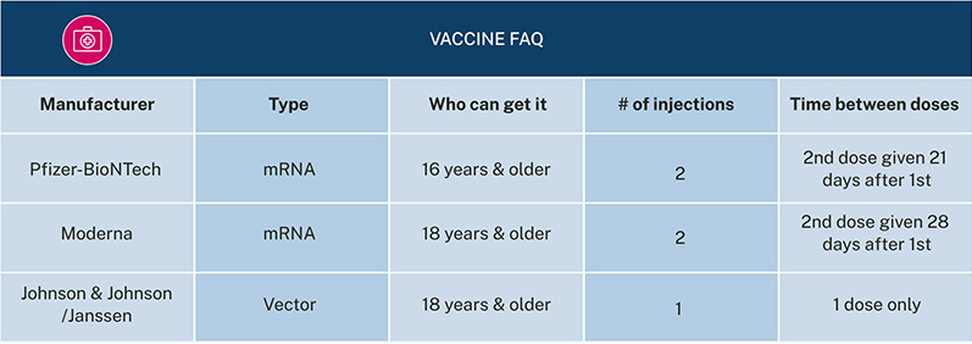
- The Pfizer-BioNTech COVID-19 vaccine uses part of the virus’ special genetic code (called messenger RNA or mRNA) to “teach” your body’s immune system how to make antibodies that defend your body against the virus that causes COVID-19. This vaccine is for people ages 16 and older. It requires two injections given 21 days apart. The second dose can be given up to 6 weeks after the first dose, if needed.
- The Moderna COVID-19 vaccine uses mRNA in the same way the Pfizer-BioNTech COVID-19 vaccine does. The Moderna vaccine is given to people ages 18 and older. It requires two injections given 28 days apart. The second dose can be given up to 6 weeks after the first dose, if needed.
- The Johnson & Johnson (J&J)/Janssen COVID-19 vaccine is a “vector” vaccine. Vector vaccines train your body to make antibodies in almost the same way the Moderna and Pfizer-BioNTech mRNA vaccines do. However, this type of vaccine uses a different type of genetic information (DNA) to “teach” your body how to defend itself against the virus. It is given to people ages 18 and older and requires one injection.
HOW COULD I BENEFIT FROM GETTING A COVID-19 VACCINE?
A COVID-19 vaccine may help:
- Prevent you from getting COVID-19, or if you get it, from becoming seriously ill or dying from COVID-19.
- Prevent you from spreading the virus that causes COVID-19 to other people, including loved ones or those who are at risk for serious illness from COVID-19.
- Help your community reach the goal of “herd” immunity. This means most people in a community have protection against the virus. Herd immunity helps prevent the spread of COVID-19.
- Prevent more variants or mutant strains of COVID-19 from developing by curbing the spread of the disease faster.
- Help everyone #CombatCOVID.
Once you’re fully vaccinated, you may be able to get back to doing some things you stopped doing because of the pandemic. For example, you can gather indoors, without masks, with other people who are fully vaccinated.
IS THE VACCINE SAFE?
The COVID-19 vaccines are considered safe and effective. They have been given Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) because researchers have closely studied the vaccines’ safety, effectiveness, and manufacturing quality. In fact, vaccines go through careful and strict testing in a lab before being tested in humans. Only vaccines deemed safe are then tested in people, starting with about 20 to 100 volunteers. Testing then expands to thousands of people before data on the safety and effectiveness of the vaccine is reviewed by the FDA. The vaccines have now been given to millions of people in the United States. The Centers for Disease Control and Prevention (CDC) and the FDA will continue to monitor the vaccines for safety, but long-term side effects are unlikely. Severe allergic reactions to the vaccine are rare. If a reaction like this happens, it will likely happen while you’re at the location where you got the vaccine; this is why you are asked to wait a little while after getting your vaccine before you leave. If this type of reaction happens, vaccine providers can quickly and effectively treat it.
None of the vaccines cause you to become infected with the COVID-19 virus. None of the vaccines affect or become part of your genetic DNA.
AM I ELIGIBLE FOR THE VACCINE?
In each state in the United States, the COVID-19 vaccine has been made available to people in phases, beginning with people who are most vulnerable to being infected with the virus, and most vulnerable to getting very sick, due to their health, age, or because they are more likely to come in contact with the virus at their job. All people ages 12 and older are eligible for COVID-19 vaccines in the United States, including all 50 states and Washington, D.C. You do not need to be a U.S. citizen to get the vaccine, and you will not be deported if you get the vaccine.
WHAT IF I'M INTERESTED IN GETTING THE COVID-19 VACCINE AND...
- …I’m at high risk for COVID-19 and I have pre-existing medical condition(s)? It’s important for adults of any age who have certain underlying medical conditions to get the COVID-19 vaccine, because they are at increased risk of getting very sick from COVID-19.
People at high risk for severe (serious) COVID-19 include:
- Older adults: Adults age 65+ are at highest risk, with adults ages 85+ at the greatest risk. More than 80% of COVID-19 deaths have occurred in people over age 65.
- People of any age, race, ethnicity, and sex with certain underlying medical conditions such as:
- Overweight (defined as a body mass index [BMI] of 25 or greater), obesity (BMI of 30–39), or severe obesity (BMI of 40 or greater)
- Current or former cigarette smoker
- Pregnancy
- Diabetes type I or II
- Heart conditions such as heart failure, coronary artery disease, cardiomyopathies, and possibly high blood pressure (hypertension)
- Chronic kidney disease
- Chronic lung diseases, including chronic obstructive pulmonary disease (COPD), asthma (moderate to severe), interstitial lung disease, cystic fibrosis, and pulmonary hypertension
- Dementia or other neurological conditions such as Alzheimer’s
- Cancer
- Down syndrome
- HIV/AIDS
- Weakened or immunocompromised immune system caused by conditions and/or treatments
- Chronic liver disease, such as alcohol-related liver disease, nonalcoholic fatty liver disease, and especially cirrhosis, or scarring of the liver
- Hemoglobin blood disorders such as sickle cell disease or thalassemia
- Received a solid organ transplant or blood stem cell transplant (includes bone marrow transplants)
- Cerebrovascular disease, such as having a stroke
- Substance use disorder such as alcohol, opioid, or cocaine use disorder
People with underlying medical conditions can get a COVID-19 vaccine as long as they have not had a serious reaction to a COVID-19 vaccine, or to any of the individual ingredients in the vaccine.
If you are considered high risk for serious COVID-19, you may also qualify for an ACTIV clinical trial for COVID-19 treatment or monoclonal antibody treatment. To learn how you can participate, please visit the Clinical Trials page or call 877-414-8106 to speak with an information specialist.
- …I’ve already had COVID-19?
Yes, you should get the vaccine even if you have already had COVID-19. The vaccine will help your body make enough antibodies against the COVID-19 virus to help prevent you from being infected again in the future. Even if you have already recovered from COVID-19, it’s possible that you could catch COVID-19 again. That’s because we don’t know yet how long a person is protected from getting sick again after they recover from COVID-19. The COVID-19 vaccine offers more help for your body to build the immunity that it might need. - …I have COVID-19 now?
If you have tested positive for COVID-19, the CDC recommends that you isolate yourself (be alone) and use precautions (social distancing, wearing a face mask, washing your hands often, etc.) for 10 days after your COVID-19 symptoms start, or 10 days after you test positive for COVID-19—whichever happens first. Before you get the COVID-19 vaccine, you should wait until you do not have any COVID-19 symptoms, have isolated yourself for 10 days, and have not had a fever for 24 hours. At that point, clinical trial results indicate it’s safe for people who have had COVID-19 to get the vaccine.
If you already have COVID-19, consider joining an ACTIV clinical trial for COVID-19 treatments. Visit the Clinical Trials page to learn more.
- …I’m pregnant?
- People who are pregnant have a higher risk of having worse COVID-19 symptoms than people who are not pregnant. If you’re pregnant, you may choose to be vaccinated. Vaccine monitoring systems for pregnancy are in place to monitor vaccine safety, and so far, they have not identified any specific safety concerns for pregnant people.
- There is no evidence that fertility problems are a side effect of any vaccine, including COVID-19 vaccines.
- If you have questions about getting vaccinated while you’re pregnant, a conversation with your healthcare provider could help.
- If you’re trying to become pregnant now or want to get pregnant in the future, you may receive a COVID-19 vaccine when one is available to you.
- …I’ve received monoclonal antibody treatment?
- If you have recently received monoclonal antibody (mAb) treatment or convalescent plasma to treat COVID-19, the CDC recommends that you wait 90 days after that treatment before you get vaccinated. This also applies if you got sick with COVID-19 and received these treatments while you were waiting for your second dose of the vaccine.
- You should also wait 90 days after you get mAb treatment or convalescent plasma before you get your second dose of vaccine. Researchers are still studying the safety and effectiveness of COVID-19 vaccines for people who have received mAbs or convalescent plasma as part of COVID-19 treatment.
HOW DO I GET THE VACCINE
You can receive your COVID-19 vaccine by scheduling an appointment online or by phone, or (in some places) by going to a drive-thru site. First, find a COVID-19 vaccine provider location near you by visiting https://www.vaccines.gov.
You may be able to receive the vaccine at a doctor’s office, hospital, urgent care center, community health center, state or local health department, mobile clinic, convention center, or cities’ public health clinics, sports stadiums, or a retail pharmacy.
You can check your state/territory and local health departments’ website for vaccine events and locations. To find your state’s/territory’s health department website, visit https://www.cdc.gov/publichealthgateway/healthdirectories/healthdepartments.html.
HOW CAN I PARTICIPATE IN A CLINICAL TRIAL FOR A NEW VACCINE AND OTHER PREVENTIONS?
Clinical trials help advance our understanding of diseases and find new ways to prevent, diagnose, and treat illnesses such as COVID-19. Multiple agencies and institutions are recruiting participants for COVID-19 vaccine and other prevention trials. Safe and effective vaccines and other preventions that work for most people are only possible when volunteers from all walks of life participate in the development of those vaccines and other preventions by joining clinical trials. If you would like to participate in a prevention clinical trial, visit https://preventcovid.org
AFTER GETTING THE COVID-19 VACCINE, IS IT SAFE TO GET OTHER VACCINATIONS?
Yes, it is safe to stay on schedule with other recommended vaccinations. Talk with your healthcare provider to find out which vaccines you or your child need and when. It is also very important to get your flu vaccine. The flu is a contagious viral respiratory illness caused by influenza viruses, and it kills between 12,000 and 61,000 people each year. Young children, older adults, pregnant women, and people with chronic health conditions are most at risk of having serious complications. The flu vaccine will lower the number of people who get the flu. This will mean less demand on healthcare resources that may be stressed by the number of COVID-19 patients.
WHAT ELSE CAN I DO TO COMBAT COVID?
It’s important for people of all ages and communities to participate in clinical trials for COVID-19 treatments. This helps us find treatments and vaccines that are safe and effective for everybody. For more information about COVID-19 clinical trials, please visit the Clinical Trials page.